Research Work Packages
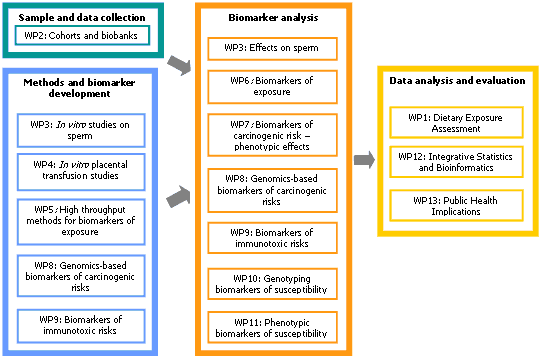
WP1: Dietary Exposure Assessment
Development of quantitative estimates of the exposure of mothers, fathers, fetuses and newborns to the chemicals of interest using questionnaires and direct analytical data.
WP2: Mother-Child Birth Cohorts and Biobanks
Organisation of the utilisation of biological material and data from 5 EU mother-child cohorts and biobanks, and establishment of an 3 new cohorts/biobanks. Identification of other EU mother-child cohorts and biobanks.
WP3: Paternal Impact
In vitro studies of the effects of exposure on human sperm and human spermatogenic cells; evaluation of semen contamination and semen quality in sub-group of available samples.
WP4: Fetal exposure
Use of in vitro placental perfusion to study in vitro/ex vivo transport of selected genotoxicants and immunotoxic substances across the placental barrier.
WP5 : Biomarkers of Exposure - Development of high throughput techniques
Development of new biomarker/bioassay techniques, including generic screening systems.
WP6 : Biomarkers of exposure- application of currently available techniques
Use of already available biomarkers (including DNA- and protein adducts, CALUX reporter gene assays) for the analysis of samples from the various biobanks.
WP7 : Biomarkers of carcinogenic risk – phenotypic effects
Analysis of maternal and umbilical cord blood lymphocytes for micronuclei and markers of apoptosis/necrosis and in vitro proliferation rates.
WP8 : Biomarkers of carcinogenic risks - genomic effects
Development of new biomarkers based on the transcriptome and proteome signatures of the chemicals of interest in maternal and cord blood cells.
WP9: Biomarkers of immunotoxic risks
Development of biomarkers of immunotoxicity through in vitro treatment of cord blood lymphocytes with the chemicals of interest.
WP10 : Genotyping markers of susceptibility
High-throughput analysis of polymorphisms encoding for susceptibility-predisposing genes.
WP11 : Phenotypic biomarkers of susceptibility
Analysis of samples from the various biobanks using a battery of phenotypic assays for DNA stability and repair, based mainly on the comet assay and its adaptations.
WP12 : Integrative Statistics and Bioinformatics
Design of protocols of biomarker studies and bioinformatics support for data analysis so as to obtain reliable effect measures, investigate exposure-response relationships and their consistency across different European regions, and to compare mother-child susceptibilities.
WP13 : Public Health Implications
Risk assessment using data on hazard identification, exposure assessment and exposure-genotype-phenotype-disease associations; derivation of public health information for decision-makers.
WP14 : Ethics and Communication
Identification of obstacles and/or possible levers to set up and conduct research on developing biomarkers in environmental health, while respecting European ethical standards and the further development of these standards.
WP15: Training
Training and educational activities among NewGeneris researchers by a) transfer of knowledge and scientific skills; b) exchange of researchers between laboratories; c) networking of existing training and research; d) practical training on the best available technologies
WP16: Dissemination
Oraganization of the flow of scientific information within the NewGeneris network and dissemination of information regarding the results of the research performed in the Project WPs, as well as relevant scientific findings from the wider scientific community, for the benefit of the European citizens, the food industry, and the policy makers.
WP17: Management
Management of the whole NewGeneris Project.